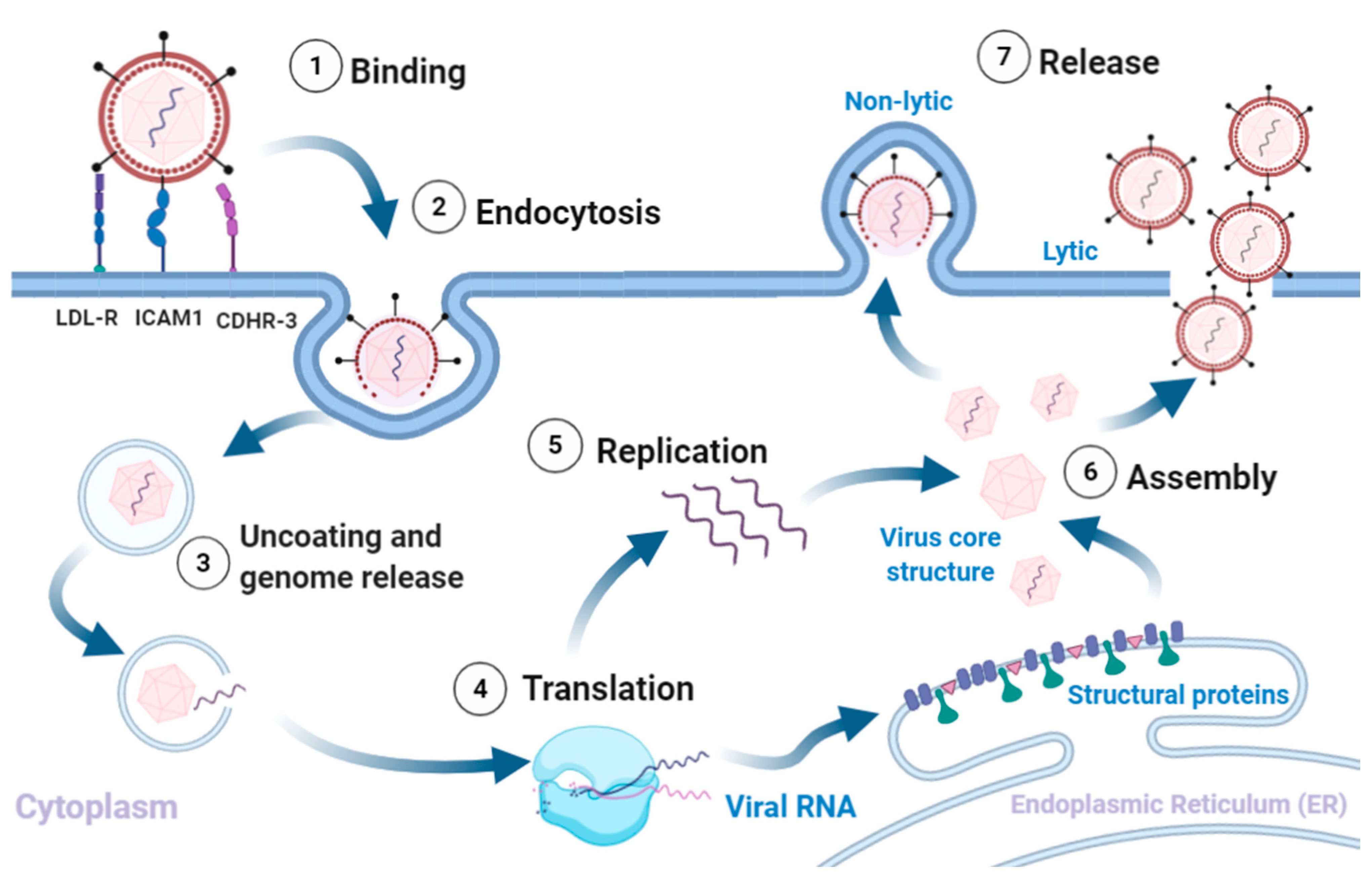
Rhinovirus
Virology
Virion Structure
Rhinoviruses are positive-sense, single-stranded RNA viruses belonging to the Enterovirus genus of the Picornaviridae family and a leading cause of upper-tract respiratory infections globally. Across the three rhinovirus species (A, B, and C), over 150 antigenically distinct rhinovirus types have been identified (Stobart, Nosek, and Moore 2017). This vast antigenic diversity is thought to be one reason why an effective vaccine has not been developed.
The rhinovirus genome is approximately 7.2kb in size and encodes 11 proteins (Figure 1) : four viral proteins (VP1, VP2, VP3, and VP4) which make up the viral capsid, and seven non-structural proteins which mediate viral replication (Palmenberg, Rathe, and Liggett 2010; Palmenberg and Gern 2014; Palmenberg 2017).
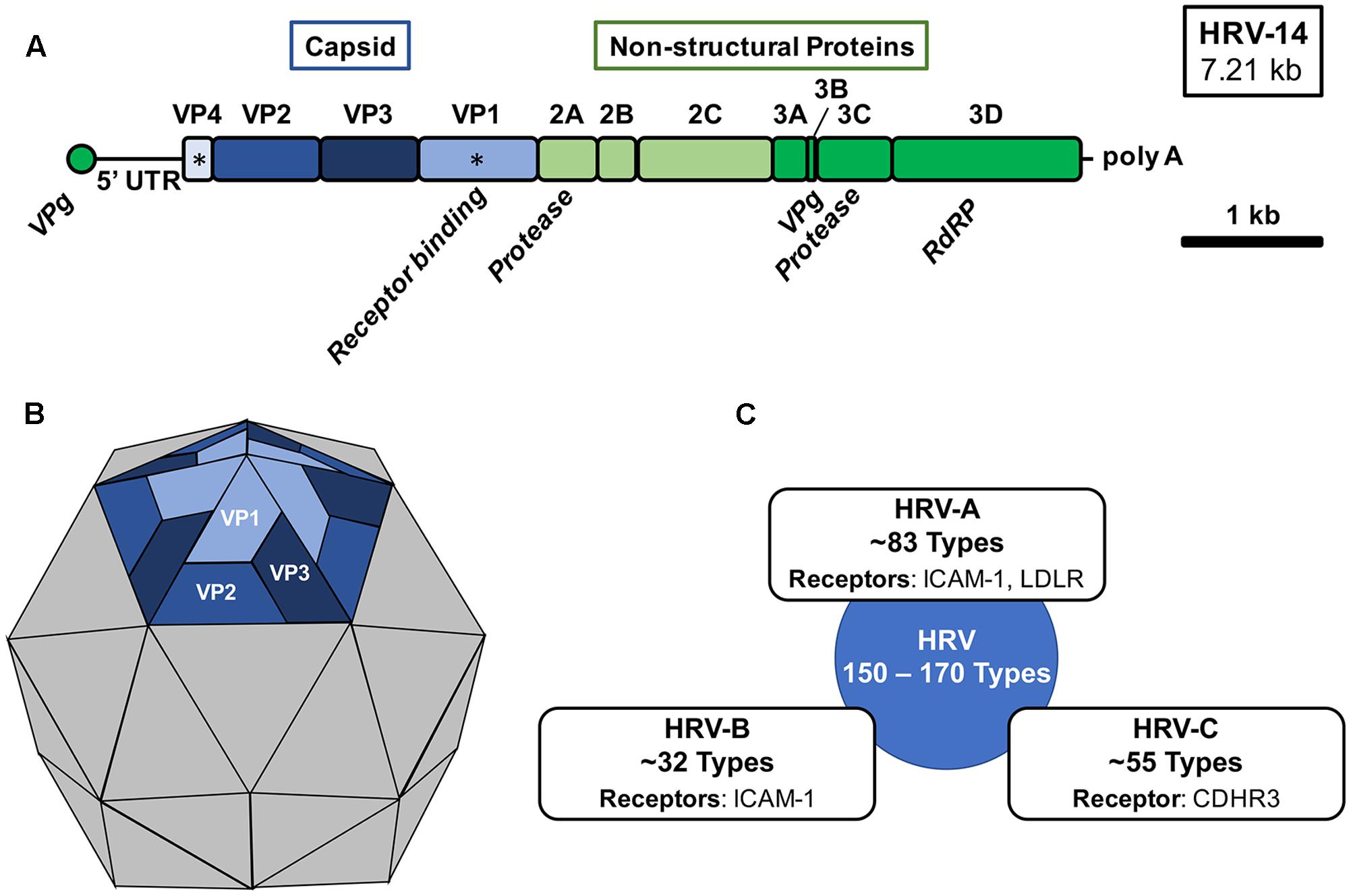
Antigenic diversity in rhinoviruses is primarily driven by the selective pressure of host immune responses against exposed surface proteins VP1, VP2, and VP3 (Stobart, Nosek, and Moore 2017).
Transmission
Rhinovirus is a highly infectious respiratory pathogen that may be transmitted via direct person-to-person contact; inoculation from an infected fomite; or inhalation of an infected aerosol (Jacobs et al. 2013). In experimental ambient conditions, rhinoviruses were shown to survive for ~2 hours on undisturbed skin and up to 24 hours on indoor surfaces (Hendley, Wenzel, and Gwaltney 1973).
Epidemiology
Symptoms
Rhinoviruses are the causative pathogen in over half of all common colds (Jacobs et al. 2013). Infection with rhinovirus most commonly occurs in the upper respiratory tract, but may also present as otitis media or sinusitis. Infection is generally mild and self-limiting. Common symptoms include:
cough
sneezing
runny nose
nasal congestion
sore throat
headache
body aches
fever
Children may also have fever in the first 2-3 days and moderate enlargement of the anterior cervical nodes. In children, elderly adults, and immunocompromised individuals, rhinovirus infection can progress into the lower respiratory tract resulting in more severe clinical manifestations such as croup, bronchiolitis, or pneumonia. Several epidemiological studies have noted an association between rhinovirus infection and the exacerbation of asthma symptoms (Ortega, Nickle, and Carter 2020). Common respiratory viruses, such as rhinovirus, are likely involved in the aetiology of asthma, but further studies are required to elucidate the exact nature of this relationship.
Disease Parameters

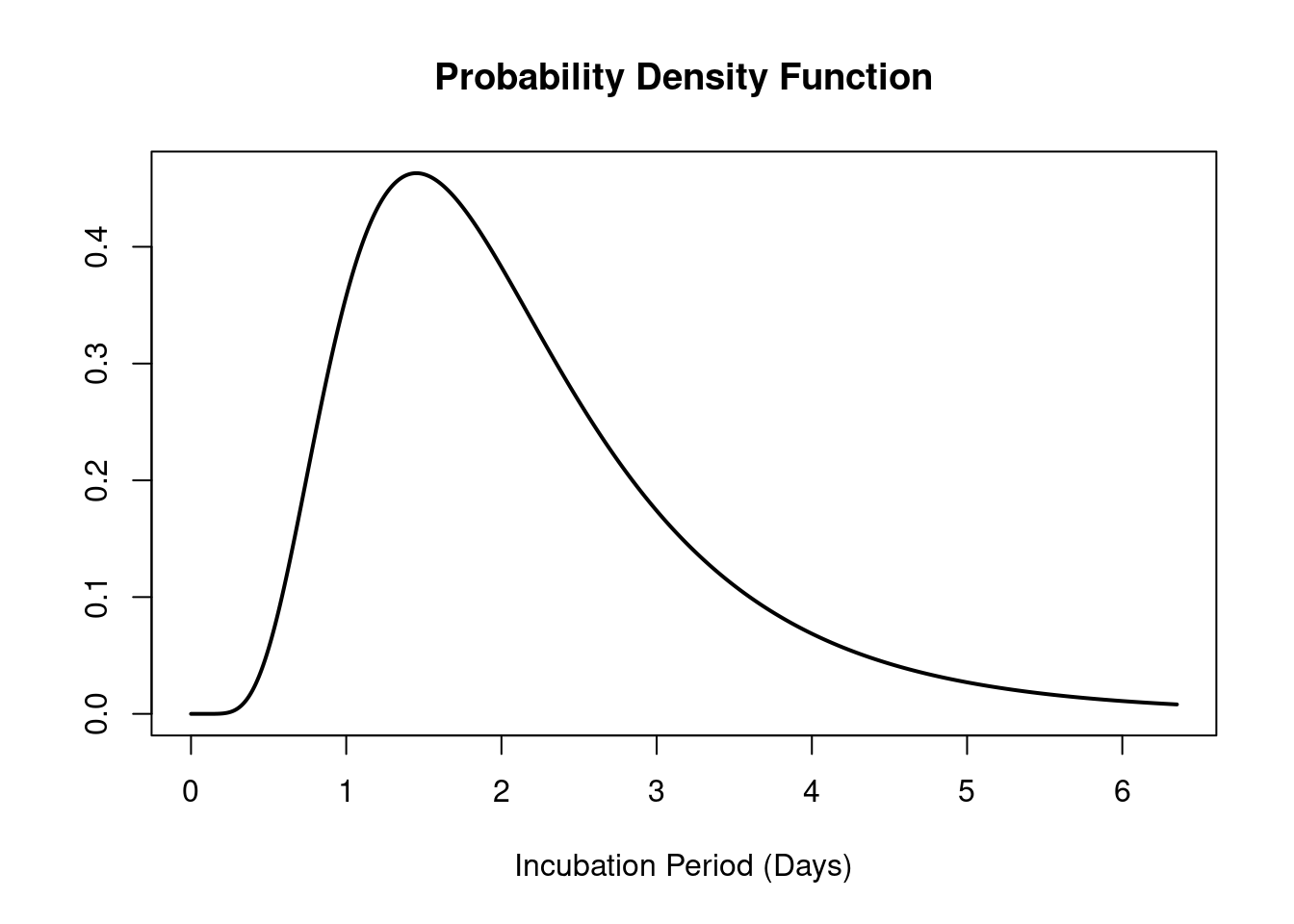
The mean incubation period of rhinovirus is ~2 days (Lessler et al. 2009; Lambert, Kucharski, and Tamayo 2025), (Figure 3). As described in the National Infection Prevention and Control Manual (NIPCM), the infectious period of rhinovirus begins a few days before onset of symptoms and may last until all symptoms have ceased. The duration of infection generally ranges from 5 to 7 days in adults, but can be up to 10-14 days in children.
Seasonality
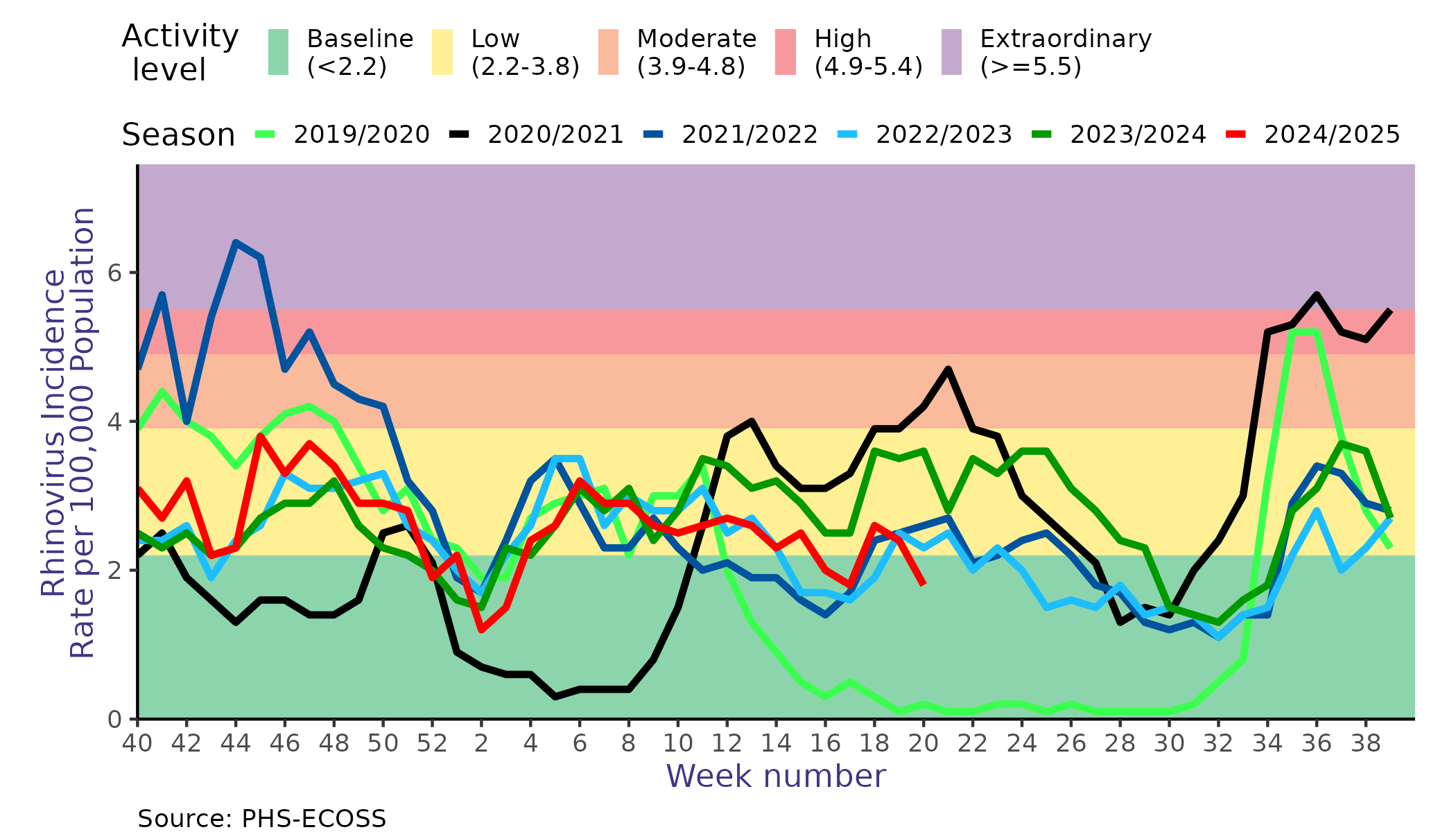
Whilst rhinoviruses are known to circulate all year round, studies in temperate climates have shown that rhinoviruses follow a biennial seasonal pattern, with a smaller peak in spring and a larger peak in late autumn (Jacobs et al. 2013). Unlike influenza and RSV, which exert their pressure during the winter months, rhinoviruses circulate at the highest levels during the spring, summer, and autumn months.
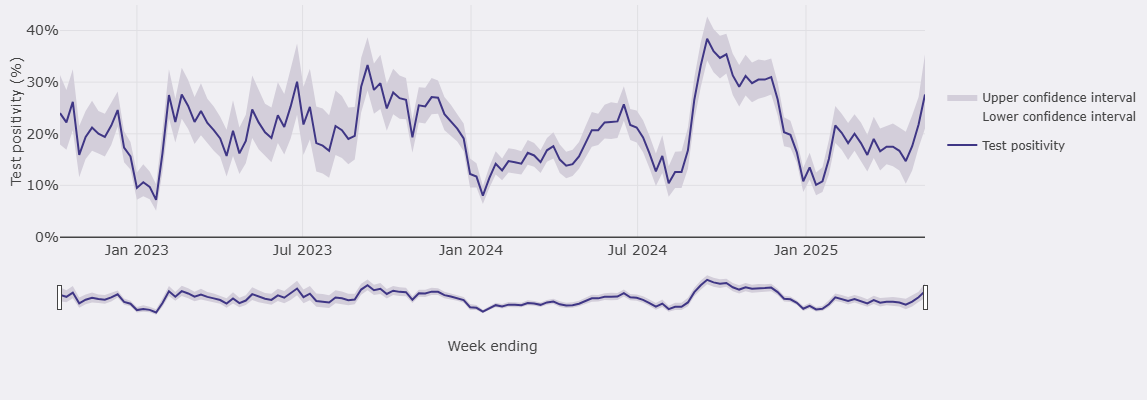
Our own data supports a spring and autumn peak, but also highlights the variability of rhinovirus activity levels (calculated using the WHO method) throughout the flu season (Figure 4).
Geographic Distribution
Rhinoviruses are globally distributed, with all species having been detected year-round in temperate, tropical, subtropical, and semiarid regions (Briese et al. 2008) .